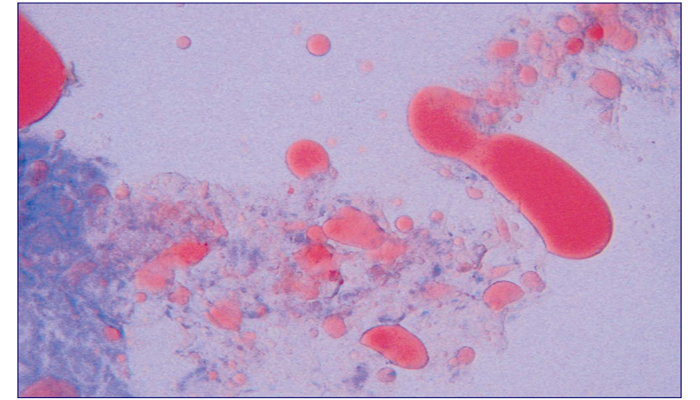
Oil Red O Stain
Intended use:
Oil red is intended for the staining of neutral fat.
Principle:
Staining of lipids is believed to relate to the physical properties of solution or adsorption. Dyes exhibit a greater solubility in lipoid substances of frozen tissue than that in original solvents. So during staining, dyes will migrate into lipids from organic solvents resulting in lipid staining.
Methods:
1. Cut frozen tissue to a thickness of 6~10μm, rinse briefly in distilled water.
2. Rinse tissue in 60% isopropanol for 20~30 seconds.
3. Prepare Oil Red O working solution with 6 mL of Oil Red O stock solution and 4 mL of deionized water. Mix well and stand for 10 minutes. Stain in Oil Red O working solution for 5~10 minutes.
4. Rinse in 60% isopropanol for several seconds to remove excess stain. Rinse in distilled water.
5. Stain nuclei in Mayer hematoxylin solution.
6. Differentiate the slides in 1% hydrochloric acid for a few seconds.
7. Blue in tap water or in diluted lithium carbonate solution for 10 minutes. Remove excess water around.
8. Mount with arabinan or glycerin jelly.
Specifications:
|
Contents |
2Btlsx250ml/kit |
Components |
|
Oil Red O stock solution |
250ml |
Oil Red O |
|
Mayer hematoxylin solution |
250ml |
Hematoxylin |
Precaution:
1. To display lipids, stain frozen tissue instead of paraffin tissue, as lipids are readily soluble in organic solvents.
2. The frozen tissue used for lipid staining should not be too thin. Otherwise, the lipid content would be lost.
3. The counterstaining time in Mayer hematoxylin should not be too long.
4. The staining will not last for too long, so observe and take pictures as soon as possible.